Introduction
The increased demand for protein as a result of the rapid growth of the world population necessitates the development of sustainable protein production methods with minimal adverse environmental effects. Novel protein sources, such as single-cell proteins, fish protein concentrates, and edible insects, are crucial for resolving protein deficiency-mediated malnutrition (Ghaly and Aloaik, 2009; Zhao et al., 2016). In particular, interest in edible insects as protein sources is growing because they have several advantages over the livestock, including species diversity, efficient production, and low environmental pollution with relatively high nutritional value (Van Huis, 2013). Accordingly, insect-based foods are consumed by over two billion people worldwide and are readily available in the US and European markets (Zielinska et al., 2018).
Entomophagy, the practice of eating insects, has been performed in various parts of the world since the emergence of humans (Ghaly and Aloaik, 2009). More than 2000 species of insects have been identified as edible. Insects are nutritious, with high protein and fat contents, biological value, and digestibility. Furthermore, insects are a source of micronutrients, including minerals and vitamins. Accordingly, insects are a potential supplement for various commercial foods (Barker et al., 1998; Rumpold and Schulter, 2013). Although whole insects are consumed in various regions, many consumers are still reluctant to accept this form. Therefore, processing into less recognizable forms may be required to increase consumer acceptability (Shelomi, 2015; Zielinska, et al., 2018).
Yellow mealworm (Tenebrio molitor L.), the larval form of the mealworm beetle, is gaining attention as an alternative protein source for various food applications. The Food and Agriculture Organization (FAO) has been estimating the potential of insects as human food and animal feed for convincing food security since 2010 (Van Huis, 2013). Many studies have reported genotoxicity, oral toxicity, nutrition composition, extraction methods, characteristics, and functional properties of proteins isolated from yellow mealworm (Finke, 2002; Han et al., 2014; Zhao et al., 2016). It is also important to understand the functional properties of proteins to improve food compositions. These functional properties, such as the water holding capacity, emulsion-forming ability, and gel formation, are dependent on physicochemical properties and pH (Zielinska et al., 2015). In addition, processing conditions, particularly heat treatment, induce conformational changes, which are associated with physicochemical properties of proteins (Lampart-Szczapa et al., 2006). Therefore, the functional properties of proteins and responses to heat treatment should be considered for practical applications.
Despite several studies of the functional properties of yellow mealworm-derived proteins, the effects of time and treatment duration are unclear. Therefore, the objective of this study was to investigate the effects of heat temperature and time on yellow mealworm-derived proteins, including physicochemical and functional properties, to provide a technical basis for their application in the food industry.
Materials and Methods
Yellow mealworm (Tenebrio molitor L.) was purchased from a commercial market (Gyeongdong market, Seoul, Korea) and stored at −80°C until use. Solvents were purchased from Samchun Pure Chemicals (Seoul, Korea). Other chemicals, including sodium dodecyl sulfate (SDS), 2-nitrobenzoic acid (DTNB), and 2,4-dinitrophenylhydrazine (DNPH), were obtained from Sigma-Aldrich (St. Louis, MO, USA).
The protein extract of yellow mealworm larva (PEYM) was prepared according to the methods described by Zhao et al. (2016), with modifications. The yellow mealworm was defatted with a 5-fold volume of ether and evaporated under a vacuum at 45°C to remove the residual ether. Protein was extracted by mixing defatted yellow mealworm larvae and 0.25 M NaOH at a ratio of 1:15 (w/v) at 45°C for 60 min. The mixture was vortexed every 15 min during the extraction procedure. After centrifugation (3,500×g, 20 min) of the slurry, the supernatant and gel fraction were separately harvested, and an additional extraction procedure was performed using the pellet. Then, 2 mol/L HCl was added to the supernatant and gel fraction, to adjust the pH to 4.3. Following centrifugation (2,500×g, 15 min), precipitated pellets were washed twice with distilled water and lyophilized. These samples were heated in centrifuge tubes in a water bath, different heat temperature treatment (55°C, 75°C, and 95°C) and different time treatment (20, 40, and 60 min). After heating, the sample of PEYM was stored at −20°C until subsequent analyses.
The solubility of PEYM was assessed according to the methods described by Zielinska et al. (2018), with slight modifications. The PEYM was suspended in distilled water, and the pH was modified to values of 2 to 11 using 6 M HCl or NaOH. The volume of each suspension was adjusted to obtain a protein concentration of 10 mg/mL. Following solubilization in 0.5 M NaOH, the suspension was stirred for 90 min. After centrifugation (8,000×g, 15 min), the protein concentration of the supernatant was assessed by the Bradford method using BSA as a standard. Protein solubility was calculated as follows:
where Ps is the protein content in the supernatant and Pt is the total protein content in PEYM.
The free thiol content of PEYM was determined according to Ellman’s method, as described by Vossen and De Smet (2015), with minor modifications. Briefly, PEYM (2 g) was added to 50 mL of sodium dodecyl sulfate (SDS) buffer (5%, w/v) and incubated at 80°C for 1 h. After cooling and filtration, 0.5 mL of each sample was mixed with 2 mL of Tris buffer (0.1 M, pH 8.0) and 0.5 mL of 2-nitrobenzoic acid (DTNB) (10 mM in 0.1 M Tris buffer). The mixture without PEYM was used as a reagent blank, and the mixture without DTNB was used as the sample blank. All mixtures were reacted in the dark for 30 min, and absorbance was measured at 412 nm. The protein content of the mixture was determined by measuring the absorbance of the sample blank at 280 nm. The protein content was calculated using a BSA-standard curve. The thiol concentration was calculated referring to the Lambert-Beer equation (ε412=11,400 M−1 cm−1), and the results are presented in nM of thiol per mg protein.
The carbonyl content of PEYM was measured as described by Vossen and De Smet (2015) using the 2,4-dinitrophenylhydrazine (DNPH) method. In particular, 1.5 g of PEYM was mixed with 15 mL of 20 mM phosphate buffer (pH 6.5) containing 0.6 mol/L NaCl. Aliquots (0.2 mL) of the mixture were mixed with 1 mL of ice-cold 10% (v/v) trichloroacetic acid (TCA) and placed in an ice bath for 15 min. Following centrifugation (2,000×g, 30 min), pellets were treated with 0.5 mL of 10 mmol/L DNPH, and the sample blank was treated with 0.5 mL of 2 M HCl. After reaction in the dark for 1 h, 0.5 mL of ice-cold 20% TCA was added. The pellets were collected by centrifugation (2,000×g, 20 min) and washed thrice with ethanol/ethyl acetate (1:1, v/v). The samples were dissolved in 1 mL of 6 M guanidine-HCl in 20 mM phosphate buffer (pH 6.5) and placed in the dark for 30 min. After centrifugation (9,500×g, 10 min), absorbance was measured at 280 and 370 nm. The relative protein concentration was calculated using BSA as standard. The carbonyl content was calculated using an absorption coefficient of 0.021 nM−1 cm−1 at 370 nm, and results are presented as nM of carbonyl per mg of protein.
The aggregation ability of PEYM was determined according to the Nile red method described by Sante-Lhoutellier et al. (2008). Briefly, 1 mg of PEYM was suspended in 1 mL of 20 mM phosphate buffer (pH 6), and 10 μL of Nile Red stock solution was added. The fluorescence intensity was measured at an excitation (λex) wavelength of 560 nm and an emission (λem) wavelength of 620 nm using a microplate spectrofluorometer. Results are expressed as arbitrary units (au).
Surface hydrophobicity was determined with the chromophore bromophenol blue (BPB) as described by Sante-Lhoutellier et al. (2008). Briefly, 2 mg of PEYM was suspended in 1 mL of 20 mM phosphate buffer (pH 6). Next, 1 mL of the sample was mixed with 40 μL of BPB (1 mg/mL in distilled water) and reacted for 10 min. Following centrifugation (2,000×g, 15 min), the absorbance of the supernatant was measured at 595 nm.
The water absorption capacity (WAC) was determined as described by Zielinska et al. (2018), with some modifications. Briefly, PEYM (0.5 g) was mixed with 20 mL of distilled water and stirred at 55×g for 30 min. After centrifugation (8,000×g, 15 min), the weight of the precipitate was compared with the initial weight. The results are expressed as g of absorbed water per g of sample.
The oil absorption capacity (OAC) of PEYM was determined according to the methods described by Zielinska et al. (2018). The PEYM (0.5 g) was mixed with 10 mL of vegetable oil and stirred for 30 s. Following centrifugation (8,000×g, 15 min), the sediment weight was compared with the initial weight. The results are expressed as g of oil absorbed per g of sample.
The emulsifying properties were assessed according to the methods described by Zielinska et al. (2018), with some slight modifications. Briefly, 0.1 g of PEYM was suspended in 10 mL of distilled water and mixed with an equal volume of vegetable oil. Following homogenization (3,500×g, 1 min), the mixture was centrifuged (3,000×g, 5 min). The height of each layer was measured. The emulsion stability (ES) was determined by heating at 80°C for 30 min. The mixture was centrifuged (3,000×g, 5 min). The emulsion activity (EA) and stability were calculated as follows:
where V is the total volume of the tube contents, V1 is the volume of the emulsified layer, and V2 is the volume of the emulsified layer after heating.
The foaming properties of PEYM were determined by the methods described by Guo et al. (2015), with slight modifications. Briefly, 0.2 g of PEYM was suspended in 20 mL of distilled water and blended using a homogenizer (2,000×g, 2 min). The sample was transferred into a cylinder, and the total volume was determined. After standing for 30 min, the foaming stability (FS) was determined. The foaming capacity and stability were calculated as follows:
where V is the volume before whipping (mL), V0 is the volume after whipping (mL), and V30 is the volume after standing (mL).
The effect of PEYM on gel strength (GS) was evaluated following the methods described by Yi et al. (2013), with modifications. Gel strength was determined using a TX-XT2 instrument (Stable Micro Systems Ltd, Surrey, UK). A spherical stainless probe (0.25 inch) was used to penetrate 90% of the PEYM gel length (10×10 mm) and the peak penetration to reach the breaking point was determined. Gel strength (g×mm) was evaluated by the force-deformation curves based on the multiplication of force (g) by distance (mm).
All experiments were conducted in triplicate, and the results are presented as means±SEM. Statistical analyses were performed using IBM SPSS 24.0 (SPSS, Chicago, IL, USA). Statistical differences were determined by one-way and two-way analyses of variance and post hoc Tukey HSD tests. A value of p<0.05 was considered statistically significant.
Results and Discussion
The results of protein solubility are shown in Table 1, solubility was significantly lower at pH values of 5 and 6 (p<0.05, respectively), than at alkaline (pH 11) and acidic (pH 2) values. Protein solubility gradually increased from pH 5 to 10 and showed a maximum of 88%. These results agree with those of Seena and Sridhar (2005), who reported that proteins have net positive and negative charges at alkaline and acidic pH values. Thus, the isoelectric point of PEYM was observed at pH 5 and 6, which was significantly affected by the heating time-temperature interaction (p<0.01 and p<0.05, respectively). The solubility increased with increasing the heating time for 40 to 60 min at each different temperature while decreased with increasing the temperature at 75°C and 95°C for each different heating time. According to Timelisena et al. (2016) and Pelegrine and Gasparetto (2005), the solubility is influenced by protein-water interaction and unfolded protein molecule at under the protein denaturation temperature and affects the binding of the secondary and tertiary structures of the polypeptide chain, resulting in hydration. On the other hand, above the protein denaturation temperature, reducing the protein-water interactions and exposure of hydrophobicity groups, resulting in decreased solubility. These results correspond with the results of previous protein studies of whey proteins solubility (Pelegrine and Gasparetto, 2005). Thus, the protein solubility results indicated that pH as well as heating time and temperature interaction influence protein solubility.
Aggregation results for PEYM are shown in Fig. 1A. The significant effects of time and the interaction on the aggregation was not detected (p>0.05). The aggregation tended to increase linearly with increasing the time, although there was not a significant difference. Aggregation increased significantly during heat temperature treatment (p<0.05) based on the increase in fluorescence as the temperature increased. Fig. 1A depicts the gradual increase in fluorescence with respect to heat and time. The most significant increase in fluorescence was observed at 95°C (64.25 au). According to Mahler et al. (2009), high temperatures affect the secondary, tertiary, and quaternary structures of polypeptide chains. During heating, aggregates form, releasing hydrophobic groups by protein-protein interactions. Our results indicated that protein aggregation increases with increasing temperatures.
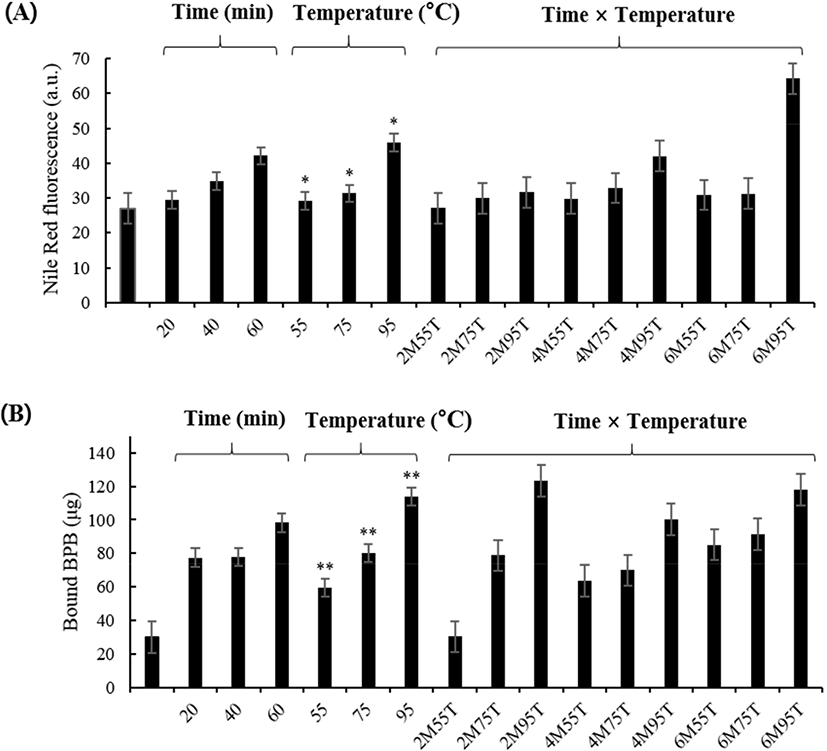
Surface hydrophobicity influences the surface characteristics of proteins, such as protein-lipid interactions and protein-protein interactions (Timilsena et al., 2016). As shown in Fig. 1B, as the temperature increased, the surface hydrophobicity increased significantly (30.35 to 118.16 μg; p<0.01); however, heating time showed no significant difference (p>0.05). Chelh et al. (2006) suggested that oxidation increases surface hydrophobicity due to the cleavage of a specific peptide chain as the heating time and temperature increase. These results are in agreement with those of Sun et al. (2013), who reported that treatment at 40°C for 60 min significantly increased (p<0.05) hydrophobicity. Thus, the increase in surface hydrophobicity suggested that the structure of PEYM changed in response to heat treatment, and the exposure of hydrophobic groups was measured.
As shown in Fig. 2A, carbonyl contents increased significantly as the temperature increased (0.93 to 1.49 nmol/mg protein) (p<0.01). At 95°C, carbonyl contents showed a tendency to increase rapidly. Previous studies have reported increases in carbonyl contents by heat and time (Roldan et al., 2014). Heat and time induce deformation by increasing oxidative stress (Sante-Lhoutellier et al., 2008). According to Roldan et al. (2014), heat and time interaction increased carbonyl compounds and, compared to time treatment, heat treatment directly affects the formation of carbonyl compounds. However, the results of this experiment did not confirm the effect of time on the carbonyl content (p>0.05). Estevez (2011) found that the formation of carbonyl compounds from amino acid chains contributes to the denaturation of a protein and decreases its functionality, which is affects protein quality. These results suggest that further studies are needed to understand the formation of carbonyl compounds with heat treatment under various time treatment.
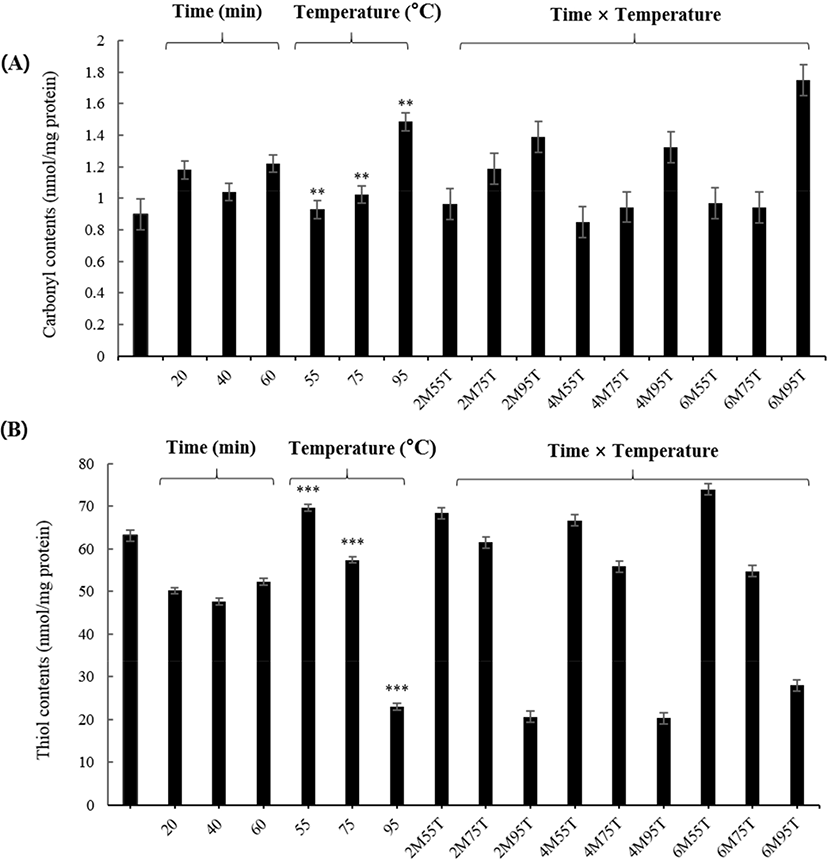
The free thiol contents are reported to decrease due to disulfide conversion during initial protein oxidation, negatively affecting the digestibility and nutritional value of food (Soyer et al., 2010). As shown in Fig. 2B, the free thiol content decreased significantly (p<0.001) as the temperature increased (69.63 to 22.94 nmol/mg protein). Heat treatment enhances the exposure of free thiol groups and the exteriorization of hydrophobic residues, resulting in the destruction of hydrogen bonds, formation of disulfides, and induction of protein aggregation (Traore et al., 2012). The results of this experiment suggest that thiol contents in PEYM are reduced by heat treatment. The thiol content of PEYM was lower than those of egg white (58.5 to 9.64 mg of protein/mL) and meat (Lund et al., 2008; Van der Plancken et al., 2005), indicating that food processing involving heat treatment at 55°C, 75°C, and 95°C is possible.
Water and protein interactions affect the functional properties and texture of food, as water absorption increases condensation and viscosity which has higher WAC, the higher the usefulness in the meat and bakery industries (Damodaran, 2017). As shown in Table 2, WAC did not differ significantly with respect to temperature and heating time (1.02 to 1.18 g/g), consistent with the results of Zhao et al. (2016) (1.29 g/g). Zielinska et al. (2018) reported that WAC could be enhanced depending on the treatment conditions for yellow mealworm protein (1.87 to 3.95 g/g). Thus, the WAC of PEYM can likely be improved by other factors, such as pH, concentration, and other extraction methods.
Indicate within a column significant difference between heating time and temperature interaction (* p<0.01, ** p<0.001). All values are expressed as means±SEM of triplicate experiments.
The result of OAC is presented in Table 2. OAC was significantly influenced by different temperatures (55°C, 75°C, and 95°C; p<0.001) and all the heating periods showed significantly lower values than those in the control group (p<0.05). OAC values decreased as the heating time increased at each different temperature treatments 55°C, 75°C, and 95°C (1.62 to 1.18, 1.66 to 1.32, and 1.74 to 1.33 g/g, respectively). Heat temperature treatment collapses the protein network and decreases OAC (Yin et al., 2008). Additionally, the formation of protein aggregates and the exposure of hydrophobic amino acid would decline the protein-oil absorption capacity (Zhao et al., 2016). These results indicated that the heating time and temperature affect the interactions of hydrophobic amino acids with oil, corresponding with the results of previous protein studies of soybean (1.1 g/g) and pea protein (1.2 g/g) (Naik et al., 2012; Shevkani et al., 2015). Furthermore, they support the use of PEYM as a food additive.
The results of EA and ES are shown in Table 2. EA and ES tended to decrease as the time increased at each different heat temperature treatments (55°C, 75°C, and 95°C). EA decreased significantly from 46.46% to 34.94% in response to the heating time-temperature interaction (p<0.001) and ES also decreased significantly from 87.59 to 69.39% in response to different heat temperature treatment (p<0.001). According to McClements (2004), the temperature exceeds the critical value and the protein unfolds exposing the internal non-polar amino groups, causing it to become insoluble or promoting protein–protein interactions. Heat denaturation also creates reactive thiol groups, promoting protein interactions and leading to changes in droplet flocculation and coalescence (McClements, 2004). Our results confirmed that EA and ES decrease by heat treatment and the observed decreases might be correlated with denaturation and droplet size. The EA results also agree with those of other studies of yellow mealworm protein (Gould and Wolf, 2018; Zielinska et al, 2018), suggesting that it is a new source of protein emulsifier for food formulations.
The results of FC and FS are shown in Table 1, FC was significantly decreased as the temperature increased (12.3% to 3.58%, p<0.001) and FS was significantly increased as time and temperature increased (88.26% to 97.37%, p<0.001). The lowest value of FC was observed at 95°C (3.58%), while the highest value of FS was observed for 60 min (97.37%). Previous studies have shown that the high sugar content in mealworms induces passive protein-protein interactions, leading to a weak foaming property due to the formation of a weak interfacial membrane during foam formation (Yi et al., 2013; Zielinska et al., 2018). According to Bals and Kulozik (2003), protein denaturation is associated with structural changes, resulting in aggregation and reactive thiol groups. The aggregates do not readily form an interfacial film. The surface load becomes smaller, and foam rigidity becomes lower, negatively affecting FC and FS. Therefore, the FC of PEYM appeared to be low and was negatively influenced by time and heat.
Gelation is induced by protein aggregation and network formation and contributes to the sensory properties and texture of food (Renkema and van Vliet, 2002). GS was significantly higher in the heating time-temperature interaction (234.21 to 2,547.37 g×mm; p<0.001). In particular, the highest GS was obtained at 95°C for 60 min. A previous study has demonstrated that heating time during gel formation affects the unfolding of protein structures and protein formation (Totosaus et al., 2002). Increasing temperatures results in gel network formation via peptide aggregation (Totosaus et al., 2002). In this experiment, protein-protein bond formation increased by time and temperature treatment. These results confirmed that gels had stronger networks than those of the control group.
Conclusion
In this study, we studied the effects of heating time and temperature on the functional properties and oxidation of PEYM. Notably, heat temperature treatment significantly influenced the surface hydrophobicity, aggregation, WAC, OAC, EA, ES, GS, carbonyl content, and thiol content of PEYM. High temperatures increased surface hydrophobicity and aggregation, induced protein oxidation, and altered the functional properties of proteins. This suggests that the heat treatment induces changes in protein properties; however, functional properties of PEYM can be maintained. Regarding the heat exposure, no significant change in functional properties and protein oxidation of PEYM were detected showing that PEYM is stable for a long time. In terms of protein denaturation (55°C to 95°C), the functional properties of PEYM were stable and appropriate for the parameters required for the food formulation and thermal processing. These results indicated that PEYM is a suitable alternative source of protein for thermal processing and the formulation of protein products. Further quantitative studies are needed to evaluate the practical applications of proteins derived from yellow mealworm larvae.













