Introduction
Oxidative stress through reactive oxygen species (ROS) can react with proteins and DNA, and damage membrane lipids through lipid peroxidation (Hernández-Corroto et al, 2018). Consequently, oxidative stress and lipid peroxidation can cause inflammation and diseases, including cardiovascular diseases, cancer etc. In the food industry, the past two decades have a growing interest in the identification of safe and natural antioxidants for dietary supplementation, to enhance the antioxidant defence system and reduce lipid oxidation of food items (Finkel and Holbrook, 2000). Recently studies for anti-oxidant extracts or peptide also had anti-proliferative effect against cancer cell lines have been elucidated (Jeong et al., 2010; Zhang et al., 2008).
Colorectal carcinoma is one of the most common malignancies and has the third highest and second highest incidence among men and women, respectively, worldwide (Ferlay et al., 2008). Colorectal carcinoma incidence has been steadily increasing in Asia, and it is associated with increased intake of Western food items and animal fat and reduced physical activity (Park et al., 2008). Currently, studies on colorectal carcinoma are focus on identifying extracts and peptides from natural sources, which have anti-proliferative properties.
Various peptides can be produced through enzymatic proteolysis of meat proteins. Such peptides can regulate and positively affect different physiological processes that may ultimately influence health, including antimicrobial action, blood pressure homeostasis (regulation of angiotensin converting enzyme activity), cholesterol homeostasis, antioxidative and antithrombotic effects (Escudero et al., 2012). In particular, beef can be considered an abundant source of bioactive peptides that can be produced during protein hydrolysis using digestive enzymes like pepsin, trypsin, or chymotrypsin (Jang and Lee, 2005). However, several epidemiological studies have reported that consumption of red meat is related with cardiovascular disease or a risen risk of cancer (Cross et al., 2007). Furthermore, the International Agency for Research on Cancer (IARC) has classified red meat as a Group 2A carcinogen (probably carcinogenic to humans) in October 2015. As per the epidemiological data obtained by the IARC, colorectal carcinoma constituted the majority of clinical cases. Controversially, Goldbohm et al. (1994) reported that red meat consumption did not related with an increase in relative rates of colorectal carcinoma, in a prospective cohort study. A previous study also has reported that peptide from beef sarcoplasmic protein hydrolysates inhibited cancer cell (Jang et al., 2008). Therefore, data regarding the association between consumption of red meat and the risk of colorectal carcinoma are still conflicting and inconsistent, along with numerous methodological limitations.
Therefore, this study aimed to isolate and purify anti-oxidant peptide from Hanwoo beef (red meat) and to determine its anti-proliferative effect against human colorectal carcinoma cell line HCT116.
Materials and Methods
At 24 h post mortem, top round (M. semimembranosus) of Hanwoo steer (quality grade 1+) purchased from a local meat-packaging centre (Chuncheon, Korea). Hanwoo beef samples (20 g) were diluted with 200 mL of distilled water (1:10 dilution) and homogenised using a Polytron PT-2500E homogenizer (Kinematica, Switzerland). Then samples were centrifuged (1,763×g, 10 min, 15℃) and filtered (Whatman No. 4). To discard fat in the samples, the filtrate (20 mL) was mixed with chloroform (6 mL). After agitating for 30 s, the mixture was centrifuged at 1,763×g at 4℃ for 10 min. The supernatant of Hanwoo beef extract (15 mL) was separated into a low-molecular-weight fraction (<3 kDa), using the centrifugal membrane filter (Amicon Ultra-3K, Millipore, USA). The low-molecular-weight fraction was lyophilized for further determination.
The lyophilised low-molecular-weight fraction was dissolved in distilled water (DW) at 50 mg/mL and separated through RP-HPLC, using a Zorbax Eclipse C18 column (4.6×250 mm) (Agilent Technologies, USA). Mobile phase A was 0.1% trifluoroacetic acid (TFA) in water (v/v). Mobile phase B was 0.1% TFA in acetonitrile (v/v) and the solvent gradient started at 0% mobile phase B and increased up to 40% for 40 min with 1.0 mL/min of flow rate. Absorbance measured at 215 nm (Agilent 1200 series HPLC system, Agilent Technologies, USA). Two-millilitre elution was lyophilized and reconstituted in 75 mM phosphate buffer (0.5 mL). It was used as sample solution for antioxidant activity.
The oxygen radical absorbance capacity (ORAC) assay was used to determining the antioxidant activity of the extracts and fractions in accordance with the slightly modified method of Gillespie et al. (2007). Briefly, 25 μL of sample solutions, or trolox (standards) mixed with 150 μL of 80 nM fluorescein. After incubating for 15 min at 37℃, 25 μL of 150 mM AAPH was added. The fluorescence of mixture recorded every minute with excitation and emission wavelengths of 480 and 520 nm, respectively, at 37℃ using a spectrophotometer (SpectraMax M2e, Molecular Devices, USA). ORAC values were determined using a regression equation between the trolox concentration and the net are under the curve (AUC). Final ORAC value was expressed as μmol of trolox equivalent (TE).
The peptides were identified using a Triple TOF 5600 Q-TOF LC/MS/MS system (ABSciex, USA) equipped with a Ultimate 3000 RSLC HPLC system (Thermo Fisher Scientific Inc., Waltham, MA, USA). Kinetex F5 column (100×2.1 mm, 2.6 μm, Phenomenex, USA) was used with gradient method. The solvent A was 0.1% formic acid in water (v/v) and solvent B was 0.1% formic acid in acetonitrile (v/v). A flow rate of 0.25 mL/min was applied and the volume of 5 μL was injected. The mass spectrometer performed under positive electrospray ionisation (ESI) ion mode and a ion spray voltage was 5,500 V. The range of full-scan mass spectra was from 50 to 2,000 m/z. The data were processed using Analyst TF 1.7 software and performed using PeakView 2.2.0.
The human colon cancer cell line (HCT116, KCLB No. 10247) was cultured in RPMI1640 (Welgen, Korea) with 10% foetal bovine serum (Welgen, Korea) and 1% antibiotics (Gibco/Thermo Fisher Scientific, USA) in a incubator containing 5% CO2, at 37℃. HCT116 cells seeded in 48-well plates (2×104 cells/well) at 37℃ for 24 h. Peptides at concentration of 0–250 μg/mL treated to HCT116 cells and incubated for 24 h. To determine cell proliferation, cell viability was evaluated using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. A MTT solution added to each well (final concentration 0.5 mg/mL) and incubated for 4 h. The generated formazan was dissolved in dimethyl sulfoxide and quantified at 540 nm using spectrophotometer (SpectraMax M2e, Molecular Devices, USA).
To determine the expression of mitogen - activated protein kinases (MAPKs) including p38 kinase, extracellular-regulated protein kinase (ERK), and c-Jun-N-terminal kinase (JNK), western blot analyses performed by previously described method (Kim et al., 2018). HCT116 cells seeded in a 6-well plate (2×105 cells/well) and incubated overnight at 37℃. The peptide added at varying concentrations into the cell cultures and incubated for 24 h. Thereafter, cells were washed 2 times with dPBS (pH 7.4) and lysed with lysis buffer (pH 8.0, 120 mM NaCl, 40 mM Tris, 0.1% NP40) containing 1x protease inhibitor cocktail (GenDEPOT, USA) on ice. Cell lysates were centrifuged at 10,000×g for 20 min at 4℃, and protein concentrations of supernatants were estimated by bicinchoninic acid (BCA) protein assay kit (Sigma Chemical Co., USA). Equivalent amounts of total cellular proteins (15 μg) were separated through sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE; 10% resolving gel) and blotted onto polyvinylidene fluoride (PVDF) membranes (Atto, Japan). For immunodetection of the proteins, membranes were blocked in 5% BSA in TBST buffer for 2 h. PVDF membranes were probed with primary antibodies (anti-ERK, -pERK, -JNK, -pJNK, -p38, -p-p38, and -β-actin antibodies at 1:200) at 4℃ overnight and post washing step to remove unbound primary antibodies the membrane was treated with secondary antibody (horseradish peroxidase-conjugated, 1:3,000–1:5,000). All primary and secondary antibodies were obtained from Santa Cruz Biotechnology (USA). The protein bands were developed using Clarity Western ECL Substrate (Bio-Rad, USA). The intensity of the bands detected with a chemiluminescence detector (AE-9150 EZ-Capture II, Atto, Japan).
Data presented as means and SEM. Analysis of variance was determined by the General Linear Model (GLM) procedure of SAS software (version 9.4, SAS Institute Inc., USA, 2013). Tukey test was used to determine the significance at p<0.05.
Results and Discussion
The low-molecular-weight fraction (<3 kDa) from Hanwoo beef extract was fractionated using a linear gradient of acetonitrile (0%–40%). Elution profile of the low-molecular-weight fraction (<3 kDa) is shown in Fig. 1A. Each 2-mL fraction was lyophilized and re-dissolved in 0.5 mL phosphate buffer and the ORAC was evaluated (Fig. 1B). ORAC values of fractions ranged from 1.76 to 70.12 μmol of TE. Fractions F1 and F2 had the highest ORAC activity and showed 63.94 and 70.12 μmol of TE, respectively (p<0.05). Therefore, F1 and F2 fractions were selected to second fractionation using RP-HPLC with a linear gradient of mobile phase B from 0% to 40% (Fig. 1C). Four fractions were further obtained and their antioxidant activity was also measured using the ORAC assay (Fig. 1D). The ORAC values of fractions P1 to P4 ranged from 22.62 to 94.21 μmol TE. ORAC value of fraction P3 was highest (94.21 μmol of TE, p<0.05) and was subjected to LC-MS/MS to identify amino acid sequence.
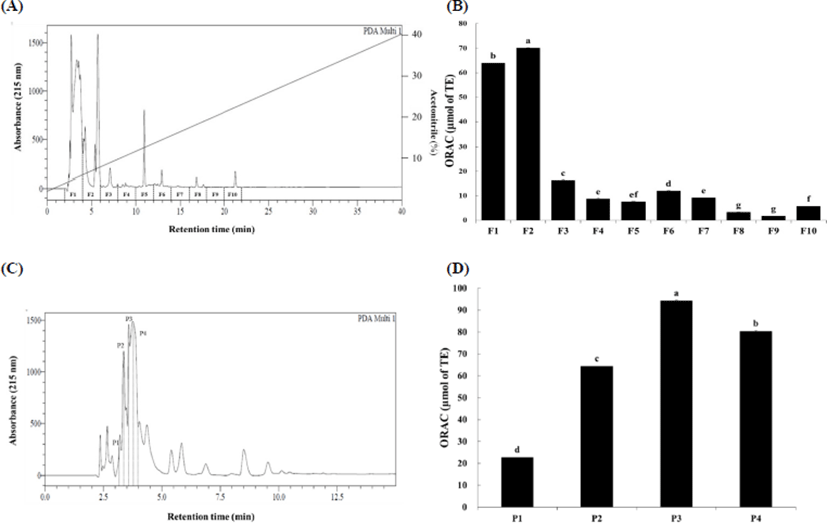
ORAC values of Hanwoo beef extract and their peptides are shown in Table 1. Hanwoo beef extract had 145.54 μM TE/g of dry matter. It has been determined that the low-molecular-weight fraction (1–3 kDa) has greater antioxidant activity than the high-molecular-weight fraction (>3 kDa) (Kim et al., 2007). The ORAC value of the low-molecular-weight fraction (<3 kDa) from Hanwoo beef extract was 167.38 μM TE/g of dry matter, which was significantly greater than that of original Hanwoo beef extract (p<0.05). Finally, we identified antioxidant peptide (P3) through RP-HPLC, which displayed the highest ORAC value at 202.66 μM TE/g of dry matter (p<0.05). These results are concurrent with those of Chang et al. (2013), wherein fraction under the 3 kDa separated displayed the highest antioxidant activity.
| Samples | ORAC (µM TE/g of dry matter) |
|---|---|
| Hanwoo beef extract | 145.54c |
| Low molecular weight peptide (<3 kDa) | 167.38b |
| Antioxidant peptide (P3) | 202.66a |
| SEM | 4.662 |
The RP-HPLC chromatogram and mass spectrogram of antioxidant peptide of P3 are shown in Fig. 2. The amino acid sequence of the antioxidant peptide (P3) and its molecular mass found to be Cys-Cys-Cys-Cys-Ser-Val-Gln-Lys and 888.30 Da, respectively (Table 2).
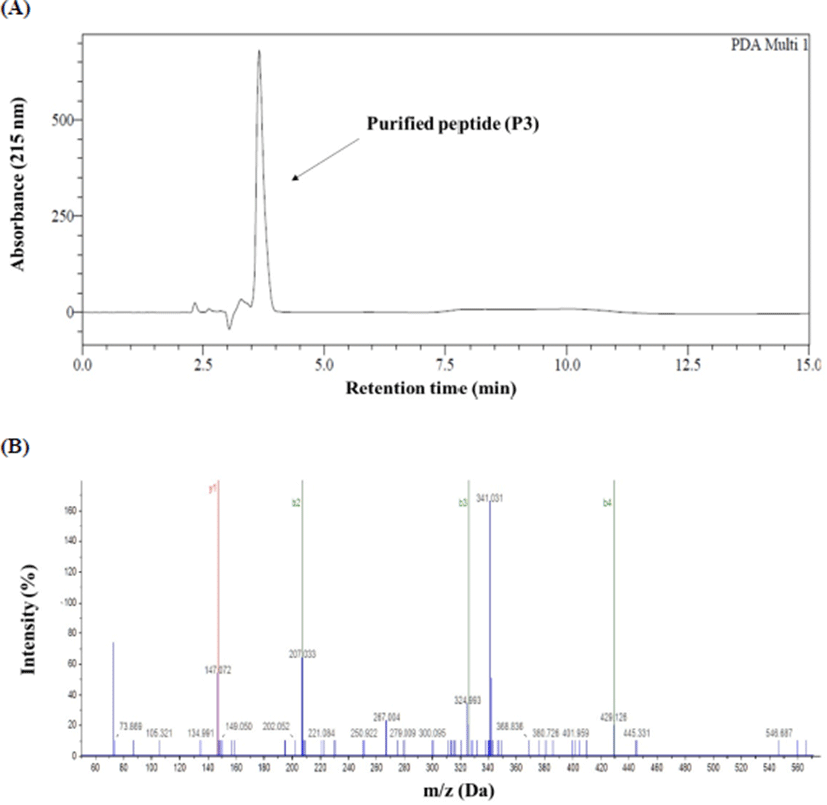
| Sample | Molecular weight (Da) | Sequence |
|---|---|---|
| P3 | 888.30 | Cys-Cys-Cys-Cys-Ser-Val-Gln-Lys |
Jang and Lee (2005) previously extracted a sarcoplasmic protein from Hanwoo rump beef (Biceps femoris) and hydrolysed it with thermolysin, proteinase A, and protease. Finally, the bioactive peptide was purified through gel filtration and RP-HPLC and its amino sequence was Val-Leu-Ala-Gln-Tyr-Lys. Seol et al. (2011) isolated peptides from Hanwoo loin (M. longissimus) during cold storage, through enzymatic proteolysis. Both studies revealed that bioactive peptide from Hanwoo beef had ACE-inhibitory activity. These data suggest that the bioactive peptides isolated from Hanwoo beef had Val, Gln, and Lys residues in their primary structure.
The function of a characterised peptide depends on sources, methods of peptide extraction, and peptide structure. Amino acid composition and size of peptide are major factors affecting their bioactivity. The numerous antioxidant peptides separated from various food proteins shows size ranging from 500 to 1,800 Da (Ranathunga et al., 2006). Small peptides easily penetrate the intestinal wall and have relatively greater biological effects. Bioactive peptides usually comprise a range from two to twenty amino acid residues, and many peptides have multifunctional properties (Timón et al., 2014). Hydrophobicity of peptides and the proportion of hydrophobic amino acids like Pro, Val, Ala, and Leu are correlated with their antioxidant activity (Wattanasiritham et al., 2016). Cys residues are known to have antioxidant effects by directly interacting with radicals; in particular, its thiol group can protect cells and biomolecules from oxidative stress (Harman et al., 1984). Acidic amino acid residues Gln and Asn can chelate metal ions by virtue of their side chains, thereby potentially inhibiting the formation of the hydroxyl radicals (Sun et al., 2016). His and Lys (basic amino acid) are well known to have antioxidant effects, which are more pronounced when incorporated into peptides (Zhang et al., 2010). Peptides purified from hydrolysed rice bran protein contained C-terminal side-chains of Lys and Arg, thereby displaying high antioxidant activity (Wattanasiritham et al., 2016). Furthermore, Chang et al. (2013) isolated an antioxidant peptide from bovine casein containing Gln-Lys at its C-terminal.
This study suggests that potent antioxidant activity of the peptide (Cys-Cys-Cys-Cys-Ser-Val-Gln-Lys) is a consequence of its amino acid composition; containing four Cys residues at its hydrophobic N-terminal, Val and Lys residue at its C-terminal.
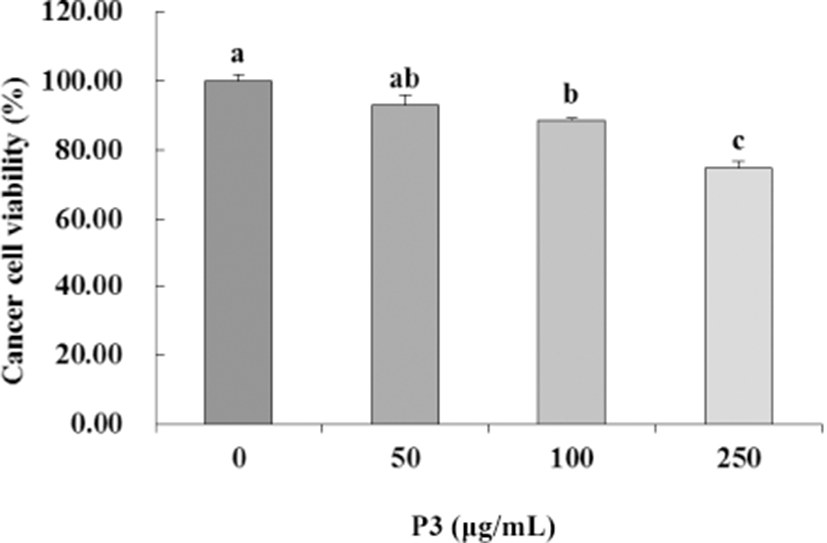
To evaluate the effect of antioxidant peptide (P3) from Hanwoo beef extract on the colorectal carcinoma cell proliferation, HCT116 cells were incubated with P3 at various concentrations (0–250 μg/mL) for 24 h. P3 inhibited the growth of HCT116 cells in a dose-dependent manner (p<0.05, Fig. 3). Inhibition of HCT116 cells was significantly greater at 250 μg/mL (74.76%) than in the control (p<0.05).
Recent studies have developed an increasing interest on the effect of diet on chronic diseases such as cancer, heart disease, and diabetes (Choi and Lee, 2009). Current studies on cancer are focus on the identification of novel compounds having anticancer properties via regulating cell proliferation and apoptosis (Liu et al., 2005). Certain peptides have been reported to display anti-proliferative effects in cancer cells, such as the tocotrienol-rich fraction from grape seeds against colorectal carcinoma and breast tumour cells (Choi and Lee, 2009), and non-digestible fractions (NDF) from the common bean against HCT116 cells (Vital et al., 2014). In particular, Jang et al. (2008) have reported antimicrobial and cytotoxic effects of peptides from beef sarcoplasmic protein hydrolysates, in human cancers. Anti-cancer peptides mediate cytotoxic effects primarily induction of apoptosis and cytoplasmic membrane destruction by pore formation or micellization (Papo and Shai, 2005).
Oxidative stress increases cancer development and progression, primarily caused by free radicals and ROS and characterised by DNA damage and protein or lipid peroxidation. Cancer cells increasingly produce free radicals compared to normal cells, further leading to cancer development (Dreher and Junod, 1996). Oxidative stress is also an important angiogenic factor; hence, established antioxidants may possibly minimize de novo angiogenesis (Monte et al., 1997). This process normally includes activation of endothelial cell and degeneration of the basement membrane, accompanied proliferation (Folkman and Shing, 1992). Therefore, reduction of oxidative damage to DNA and cells is a critical factor in preventing carcinogenesis (Zhang et al., 2008).
Numerous studies have reported both antioxidative and anti-proliferative properties of protein extracts or peptides in cancer cell lines. Choi and Lee (2009) reported that a tocotrienol-rich fraction obtained from grape seeds had high 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical scavenging activity, greatly inhibited lipid peroxidation, and had higher anti-proliferative activity against breast and colon tumour cells. Black raspberry extract had high capacities for scavenging 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ABTS radicals and also inhibited proliferation of HT-29 colon cancer cells in a dose-dependent manner (Jeong et al., 2010). Zhang et al. (2008) suggested that phenolic compounds isolated from strawberry had high antioxidant activities and inhibited cell growth of human cancer cell in a dose-dependent manner. However, the mechanism underlying the antioxidant activity against cancer cells remains unclear. Some studies have attempted to determine the mechanism underlying the antioxidant effects of peptides on cancer cells. Umayaparvathi et al. (2014) reported that peptides isolated from oyster (Saccostrea cucul-lata) protein hydrolysates had high scavenging activity on DPPH and ABTS radicals and cytotoxicity effects on HT-29 cells through induction of apoptotic morphological changes and oxidative DNA damage. Similarly, the peptide P3 was characterised on the basis of antioxidant and anti-proliferative activities against HCT116 cells.
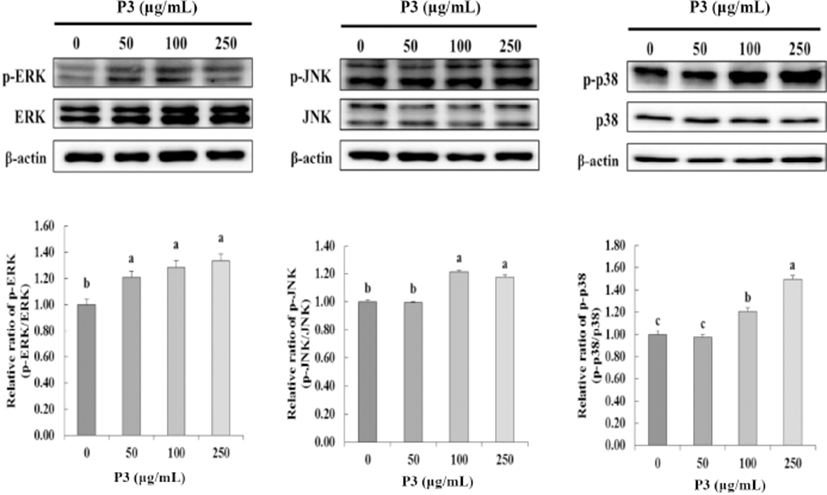
MAP kinases, including p38, ERK, and JNK kinase, play a central role in regulating cell proliferation, differentiation, and apoptosis (Stadheim and Kucera, 2002). To determine the effect of antioxidant peptide P3 from Hanwoo beef extract on MAP kinase activation, P3 treated to HCT116 cells at 0, 50, 100, and 250 μg/mL. As shown in Fig. 4, ERK phosphorylation activated by P3 at 50 μg/mL (p<0.05). Furthermore, P3 significantly activated JNK phosphorylation at 100 and 250 μg/mL (p<0.05). Antitumor compounds reduced cancer cell viability via regulating the MAPKs expression in most cancer cell lines. Especially, in this study, p38 significantly phosphorylated by P3 with dose dependent (p<0.05). The P3 inhibited cell viability (Fig. 3) and phosphorylated p38 at the same dose (100 ug/mL and 250 ug/mL) (Fig. 4). From these results, we assume that the key MAPK for inhibition of cancer cell viability was p38.
Tumor cells have an uncontrolled cell cycle, leading not to regulate cell proliferation (Kao et al., 2013). Antitumor agents mediate their effects via cell cycle arrest, cytotoxicity, anti-inflammation and, antioxidant pathways. The numerous molecular factors, including ERK, JNK, p38 kinase, have been associated with colon cancer progression (Agarwal et al., 2006). In particular, MAP kinases regulate physiological processes in cancer cells, such as cell growth, differentiation, and apoptosis (Ahmed-Choudhury et al., 2006). Numerous studies suggest that antitumor extract such as gingerol (Ryu and Chung, 2015), water extract of Gleditsia sinensis thorns (Lee et al., 2010), and Oridonin (Ren et al., 2016) can regulate MAP kinase activation and induce apoptosis in most cancer-cell lines.
Peptides isolated from various materials having anticancer effects have reported. The Azufrado Higuera cultivar of common bean NDF displayed anti-proliferative effects against HCT116 cells by changing the expression of markers for cell cycle arrest (Vital et al., 2014). The bioactive peptide (Leu-Ala-Asn-Ala-Lys) from oyster protein hydrolysate induced cancer cell death via inhibiting cell growth and causing apoptosis in HT-29 cells (Umayaparvathi et al., 2014). A tripeptide extracted from Sepia esculenta with the sequence Gln-Pro-Lys (343.4 Da) inhibited human prostate cancer cell proliferation by inducing apoptosis and regulating markers related to mitochondrial apoptosis pathways, such as Bcl-2 and Bax (Huang et al., 2012). These studies reported that peptides having anti-cancer and antioxidant effects have a conserved Lys at the C-terminal. Similarly, the peptide isolated from Hawnoo beef extract in this study had a conserved Lys residue at its C-terminal. Therefore, its structural similarity and traits may enhance its anti-cancer and antioxidant effects.
Although antioxidant pepetide (Cys-Cys-Cys-Cys-Ser-Val-Gln-Lys) from Hanwoo beef extract has anti-proliferative effect against human colon cancer cell, there is need for future in vivo studies before using these peptide for food industry.
Conclusion
Antioxidant octapeptide (Cys-Cys-Cys-Cys-Ser-Val-Gln-Lys; 888.60 Da), containing four Cys and Lys residues at the C-terminal, was identified and isolated from Hanwoo beef extract. This purified peptide had high oxygen absorbance capacity. Furthermore, the peptide had anti-proliferative effects on HCT116 cells via phosphorylation of ERK, JNK, and p38 kinase at 100 and 250 μg/mL. The present results suggest that antioxidant peptide P3 isolated from Hanwoo beef extract can be useful as a potential nutraceutical and antioxidant-based anticancer agent. These results constitute valuable scientific evidence to obliterate misconceptions regarding red meat consumption and carcinogenesis in humans.













