Introduction
Alkaline phosphatase (ALP), a protein first reported by Suzuki et al. (1907), is one of over 60 endogenous enzymes present in raw bovine milk (Schlimme et al., 1997). Since ALP is inactivated by thermal treatment, reduced levels of ALP activity are an indicator of adequate milk pasteurization (Rankin et al., 2010). The ALP assay is widely used and recognized as the most appropriate indirect method for verifying complete milk pasteurization due to its rapidity and sensitivity (Rankin et al., 2010).
The United States Food and Drug Administration (FDA) outlines quantitative criteria for ALP levels in cheese products (United States Food and Drugs Administration, 2001); the levels can be measured with a spectrophotometer and are expressed as micrograms of phenol per gram of cheese (United States Food and Drugs Administration, 2001). In contrast, Korea uses a qualitative standard for determining ALP levels in pasteurized milk products (Korea Food and Drug administration, 2015), and the results are compared visually against a set of standards (Korea Food and Drug administration, 2015).
The results of such a visual colorimetric method can be easily misinterpreted, which poses a huge public health risk, since contaminated milk is an important vehicle for the foodborne transmission of numerous pathogens (Klotz et al., 2008). Therefore, the interpretation of the results of ALP assays for milk samples should be improved. The aim of this study was to establish quantitative standards for ALP in pasteurized milk by using a spectrophotometric method.
Materials and Methods
Raw bovine milk was kindly provided by a cow farm located in Gyeonggi province and stored at 4℃ for 1 h. The raw milk was divided into conical tubes in 10 mL aliquots and subjected to heat treatment for 0, 10, 20, 30, and 40 min at 63℃ using a heat block. The heat-treated milk samples were used to determine ALP cut-off values in properly pasteurized milk. The determined cut-off value was verified using seven low-temperature and five ultra-high temperature (UHT; heat treated for 3 sec at 135℃) pasteurized milk products purchased from a retail market in Seoul, Korea. In addition, the raw milk was heat treated for 5 min at 95℃, regarded as high-temperature short-time (HTST) pasteurized milk.
ALP levels in the milk samples were analyzed using a spectrophotometer (Multiskan FC; Thermo Fisher Scientific, USA) following a method outlined in the FDA’s Bacteriological Analytical Manual (United States Food and Drugs Administration, 2001). Two 0.5 mL samples (test and boiled control samples) were mixed with 0.5 mL of distilled water. Controls were heated in a boiling water bath for 2 min and then rapidly cooled in an ice bath. Five milliliters of 2-amino-2-methyl-1-propanol (AMP) buffer substrate were added to both the test and boiled control and mixed by vortexing. Test samples and boiled controls were then immediately incubated in a 40℃ water bath for 15 min. Subsequently, the test samples were mixed with 0.2 mL 2,6-dichloro-quinonechloroimide (CQC) catalyst solution. The mixture was immediately placed back in the 40℃ water bath for 5 min and then cooled in an icewater bath for 5 min. Next, 3 mL of butanol was added to the samples and mixed by inverting the tubes six times. The mixtures were chilled in an ice-water bath for 5 min and then centrifuged for 5 min at 2400 g. The butanol (upper) phase was transferred to a new test tube. The absorbance of the butanol extracts was measured using a spectrophotometer at 650 nm.
The ALP Standard curve was created using a phenol standard solution according to the BAM method (United States Food and Drugs Administration, 2001). One gram of pure phenol was added to 1 L of 0.1 N HCl and gently mixed. One hundred microliters of the solution were added to 100 mL of AMP buffer, and gently mixed. Finally, 0, 0.25, 0.5, 1, 2.5, and 5 mL of the solution were added to 5 mL of AMP buffer and then 0.5 mL of water was added to each tube. These dilutions were used to generate the standard curve. The phenol standard solutions were incubated in a water bath for 15 min at 40℃. Subsequently, the test samples were mixed with 0.2 mL CQC catalyst solution. The mixture was immediately placed back in the 40℃ water bath for 5 min then cooled in an ice-water bath for 5 min. Next, 3 mL of butanol were added to the samples and mixed by inverting the tubes six times. The mixtures were chilled in an ice-water bath for 5 min and then centrifuged for 5 min at 2400 g. The butanol (upper) phase was removed to a new test tube. The absorbance of the butanol extracts was measured using a spectrophotometer at 650 nm. The standard curve was plotted as μg phenol against absorbance and a linear function equation was calculated.
The absorbance of milk samples measured with a spectrophotometer was converted into μg phenol/mL values using the standard curve. The μg phenol/mL milk value of the boiled blank was subtracted from the corresponding sample providing the final μg phenol/mL milk for the sample.
All experimental procedures were repeated five times. Microsoft Excel 2010 (Microsoft Co., USA) was used for data analysis. The μg phenol/mL milk value of each sample was analyzed for statistical significance using a Student’s t-test. Differences were considered significant for p<0.05.
Results and Discussion
Raw milk, a highly perishable, potentially hazardous food source, has been recognized as a significant vehicle for the foodborne transmission of many pathogens (Rankin et al., 2010). Many regulatory bodies have consequently mandated that milk be subjected to thermal treatment (Klotz et al., 2008). ALP is an established indicator for the adequacy of this pasteurization treatment.
1)Different letters in the same column indicate statistical differences (p<0.05, Student’s t-test).
2)Values < 0 were recorded as 0.
Colorimetric, fluorometric, chemiluminescent, and immunochemical methods have been employed to detect residual ALP activity for decades (Rankin et al., 2010). In dairy industry, the colorimetric assay is the most commonly and broadly adopted to verify pasteurization adequacy (Rankin et al., 2010). Despite many advantages of being rapid, inexpensive, and convenient, colorimetric ALP assays might be unreliable especially in samples with ALP levels of near the detection limits, because the interpretation depends on subjective visual assessments of color development (Murthy et al., 1992). Consequently, it is needed to develop a more objective methodology to confirm the results of colorimetric ALP assays (Murthy et al., 1992).
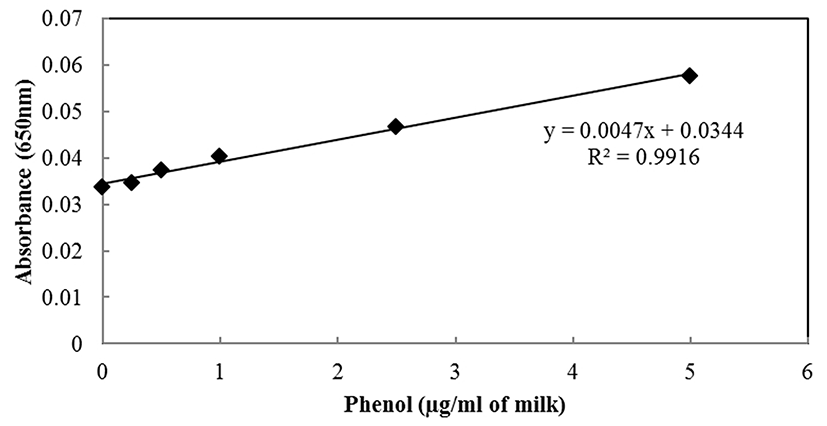
In order to quantify the residual ALP level in milk samples, a standard curve was generated using a phenol standard solution (Fig. 1). The linear function equation for the ALP standard curve was y = 0.0047x + 0.0844 (x, μg phenol/mL milk; y, absorbance at 650 nm) and the correlation coefficient (R2) was 0.9916.
Significant differences were apparent among the levels of residual ALP in the milk samples heat-treated for 0, 10, 20, 30, and 40 min. Since the legal definition for pasteurization is 63℃ for 30 min, the cut-off value was set at 2 μg phenol/mL of milk, which is just above the ALP value of milk samples heat-treated for 30 min.
In addition, the mean value of residual ALP in HTST-pasteurized milk was 0 μg phenol/mL milk. The residual ALP level of seven commercial low-temperature and five UHT-pasteurized milk products was tested using the determined cut-off value. The mean value of residual ALP in commercial pasteurized milk products was 0 μg phenol/mL milk; none of the tested milk samples demonstrated levels higher than the cut-off value, suggesting that all tested milk products were properly pasteurized.
According to the BAM, the quantitative criteria for residual ALP in cheese products differ by cheese type (United States Food and Drugs Administration, 2001); the maximum levels range from 12 to 20 μg phenol/g cheese. This cut-off value is higher than the value determined in the present study. This might be due to cheese containing bacterial starter cultures, which could provide a source of ALP (Fanni, 1983; Hammer and Olson, 1941). In addition, according to the AOAC official methods, the criterion for residual ALP in milk is 4 μg phenol/mL of cow milk (AOAC international, 2000). This is less stringent than the 2 μg phenol/mL of milk value determined in this study, suggesting that quantitative criteria could improve the safety of milk products.
The results of the present study provide basic information regarding the relationship between the duration of heat-treatment and residual ALP levels, which could aid in establishing quantitative criteria for residual ALP in milk products, thereby facilitating microbiological quality control of dairy products and improving public health.













